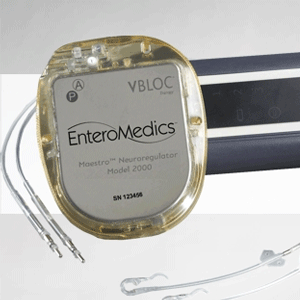
US regulators have approved a new kind of pacemaker-like device that aims to help people lose weight by stimulating a nerve that runs from the brain to the stomach.
Electrodes in the abdomen
The Maestro Rechargeable System is made by Minnesota-based EnteroMedics, and is the first device for weight loss approved by the US Food and Drug Administration in eight years.
Patients are surgically implanted with electrodes in the abdomen, and an externally-controlled electrical pulse generator sends signals to the abdominal vagus nerve, which helps signal to the brain if the stomach feels empty or full.
The FDA said the electrical stimulation "blocks nerve activity between the brain and the stomach", but that the "specific mechanisms for weight loss due to use of the device are unknown".
The device failed to meet its goal in a study of safety and effectiveness, in which it helped people lose 8.5 percent more of their excess weight than a control group that was given the device but it was not activated.
The goal had been 10 percent. The clinical trial included 233 patients with a body mass index (BMI) of 35 or greater.
Read: When to consider weight loss
However, about half of the patients that received the activated device lost at least 20 percent of their excess weight.
Watch: Patient Mike Magnant shares his weight loss success resulting from effective VBLOC® therapy.
Post approval study
An advisory committee to the FDA recommended the device's approval based on 18 months of study that showed it could help some patients lose weight and keep it off.
Read: Is Fexaramine the weight loss pill we've all been waiting for
"As part of the approval, the manufacturer must conduct a five year post approval study that will follow at least 100 patients and collect additional safety and effectiveness data including weight loss, adverse events, surgical revisions and explants and changes in obesity-related conditions," the FDA said.
Serious side effects have included nausea, pain at the neuroregulator site, vomiting, and surgical complications. Some users have also reported pain, heartburn, problems swallowing, belching, mild nausea and chest pain.
Christopher Ochner, an obesity and nutrition expert at The Mount Sinai Hospital in New York, said the device's approval is a "positive step".
Read: Obesity and diseases
"The vagal nerve, on which this system is designed to impart electrical impulses, is quite important in regulating communication along the gut-brain axis," he said.
"We have come to realize just how vital the neurohormonal system is to the regulation of food intake and body weight, and therapies that alter signalling within this system are almost certainly the wave of the future."
The FDA has now approved a total of three devices for weight loss, including the Lap-Band Gastric System and the Realize Gastric Band. Both work by limiting how much food can fit in the stomach.
Read more:
US health insurers to cover weight-loss surgery?
Bariatric surgery substantially reduces the risk of diabetes
'Love your body' to lose weight




 Publications
Publications
 Partners
Partners










