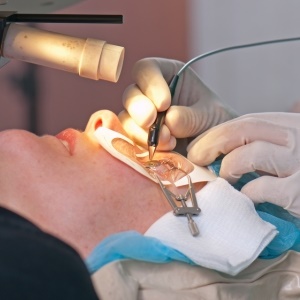
An implant that helps the ageing eye focus on small print and nearby objects has been approved by the US Food and Drug Administration.
Vision correction
The implant is placed in the cornea (the clear, front surface) of one eye in people with presbyopia, an age-related loss of the ability to change the focusing power of the eye.
"Given the prevalence of presbyopia and the ageing of the baby boomer population, the need for near vision correction will likely rise in the coming years," Dr William Maisel said in an agency news release. Maisel is deputy director for science and chief scientist in the FDA's Centre for Devices and Radiological Health.
Read: Africa needs a sustainable solution for cataract blindness
The implant resembles a tiny contact lens smaller than the eye of a needle. It is approved for use in people aged 41 to 65 who have not had cataract surgery, can't focus clearly on near objects or small print, and require reading glasses with +1.50 to +2.50 diopters of power, the FDA said. But these patients do not need glasses or contacts for long-distance vision.
To implant the device, an eye surgeon creates a flap in the cornea of the patient's non-dominant eye with a laser, slides the device into the opening, and puts the flap back in place.
"The Raindrop Near Vision Inlay provides a new option for surgical, outpatient treatment of presbyopia," Maisel added.
Can worsen problems
This is the second FDA-approved cornea implant for correction of near vision in people who have not had cataract surgery, and the first implant that changes the shape of the cornea to improve vision, according to the news release.
Read: Is laser eye surgery painful?
The FDA's approval of the Raindrop implant – made by California-based Revision Optics, Inc. – is based on a clinical trial of more than 300 patients. Two years after receiving the implant, 92 percent of the patients were able to see with 20/40 vision or better at near distances with the implant, according to the FDA.
The implant can cause or worsen problems with glare and halos, the FDA added. And there is a risk of developing infections that may cause complications of the cornea, such as corneal scarring, swelling, inflammation, thinning, clouding or melting. Some patients may require a second surgery to remove or replace the inlay, the agency said.
Read more:




 Publications
Publications
 Partners
Partners











