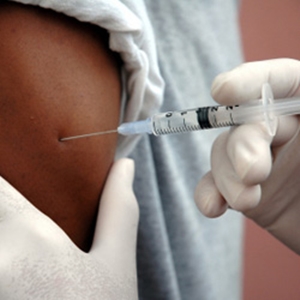
Safety trials on an Ebola vaccine are being fast-tracked, meaning it could be given to healthy volunteers as early as September, researchers said Thursday.
The move was announced by pharmaceuticals giant GlaxoSmithKline, which is jointly developing the vaccine, and Britain-based medical charity Wellcome Trust, which is contributing to a grant for the trials.
"A candidate Ebola vaccine could be given to healthy volunteers in the UK, the Gambia and Mali as early as September, as part of a series of safety trials of potential vaccines," a statement said.
"Human trials of this candidate vaccine, being co-developed by the US National Institutes of Health (NIH) and GlaxoSmithKline, are to be accelerated with funding from an international consortium in response to the Ebola epidemic."
A total of 1,552 people have died from the Ebola outbreak which erupted earlier this year in west Africa.
Read more:
Scientists are racing to test Ebola vaccines on humans
No licensed drugs or vaccines for Ebola – yet
Clinical trial of GSK Ebola vaccine to start soon




 Publications
Publications
 Partners
Partners










