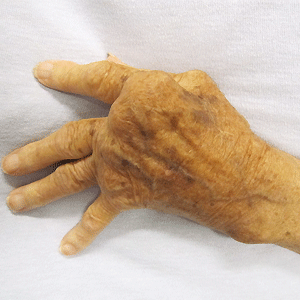
Nine years ago an experimental drug tested on nine men caused appalling side effects in a clinical trial.
One patient's head swelled to abnormally large proportions and his limbs suffered discoloration. Another patient suffered certain overgrowths, prompting comparisons with Joseph Merrick, the so-called Elephant Man.
The small German-Russian biotech firm which now owns the drug hopes to confirm its benefits in a mid-stage trial, starting in June, and then bring in a major pharmaceutical company as a strategic investor.
It is a remarkable turnaround from March 2006, when six healthy volunteers given the drug in London ended up in intensive care. One of them was described as looking like "the elephant man" after his head ballooned. Another lost his fingertips and toes.
The disaster led to the collapse of Germany's TeGenero, the initial developer of the medicine known as TGN1412.
Since then, however, scientists have developed a way to study the effects of the drug on human cells in a test tube, allowing the rational calculation of a safe starting dose.
That has provided a way back into clinical testing for TheraMAB LLC, a start-up company operating in Germany and Russia, backed by Moscow-based fund Bioprocess Capital Ventures, which bought the rights to TGN1412 and renamed it TAB08.
According to an editorial to be published in the next edition of the British Journal of Clinical Pharmacology, TAB08's "potentially triumphant return" shows that high-risk medicines can be tested safely in humans if researchers have detailed insight into how they work.
"In the course of this disaster a couple of people almost died, the company (TeGenero) went under and investors lost their money," journal editor Adam Cohen of Leiden University told Reuters. "None of that should have happened."
There is a still a long way to go and the drug may yet fail in later-stage studies or prove uncompetitive against existing treatments.
But if successful, its comeback could echo the story of thalidomide, which caused birth defects when it was launched half a century ago as a treatment for morning sickness in pregnancy and has now been repurposed as a treatment for blood cancer.
Take the test: Do you know these toxic substances in our environment?
The monkey test pitfall
TheraMAB Chief Executive Dmitry Tyrsin said in an interview he had already discussed the project with more than 20 potential "big pharma" partners, though any deal would depend on results from the upcoming mid-stage Phase IIa trial, involving 120 to 150 patients.
That trial has regulatory approval to start in Russia in June and Tyrsin hopes to get approval to extend it to the United States later this year. Further off, he aims to test TAB08 in two more autoimmune diseases, lupus and psoriasis.
TAB08 is a so-called CD28 superagonist which works by activating components of the immune system called regulatory T cells, rather than blocking the system as happens with existing antibody medication for rheumatoid arthritis.
Read: Treatment for rheumatoid arthritis
That brings a risk of over-stimulation, which is what happened in the London trial in 2006 when scientists failed to realise that successful tests on cynomolgus monkeys were not relevant for humans because of subtle immune system differences.
Now, armed with results from the new lab test, TheraMAB is using doses that are 10 to 20 times less than in the London study. It has found early signs of long-lasting benefit in reducing arthritis symptoms in small initial Phase I tests in people, with no serious side effects.
In the high-risk biotech industry, the odds of translating such early success into a commercial product remain low, but Tyrsin believes the resurrection of TAB08 has wider implications.
"This is good news for the whole industry," he said. "It shows that if you plan pre-clinical development in the right step-wise way, then you can safely develop potentially risky drugs."
CD28 superagonists are also being investigated by a number of academic groups in Europe and Asia for various inflammatory conditions, as well as stroke and heart disease. None of these programmes has yet been tested in humans.
Read more:
Experimental drug cures Ebola in monkeys
Chinese herb for rheumatoid arthritis
Inflammatory vs. mechanical arthritis
Image: By James Heilman, MD (Own work), via Wikimedia Commons




 Publications
Publications
 Partners
Partners










