TB Alliance and its partners have announced the launch of "STAND", a global Phase 3 clinical trial of a drug regimen, which shows the potential to improve multi-drug resistant tuberculosis (MDR-TB) treatment by being dramatically shorter, simpler, safer, and more affordable than current standard therapy.
STAND promises to improve treatment
The drug regimen also holds promise to improve treatment for those with drug-sensitive TB and those with TB/HIV Aids co-infection. Results of an earlier study of the regimen were published today in The Lancet.
The STAND (Shortening Treatments by Advancing Novel Drugs) trial will include approximately 50 study sites across Africa, Asia, Caribbean, Eastern Europe, and Latin America. This includes 13 sites in South Africa, where the trial was started.
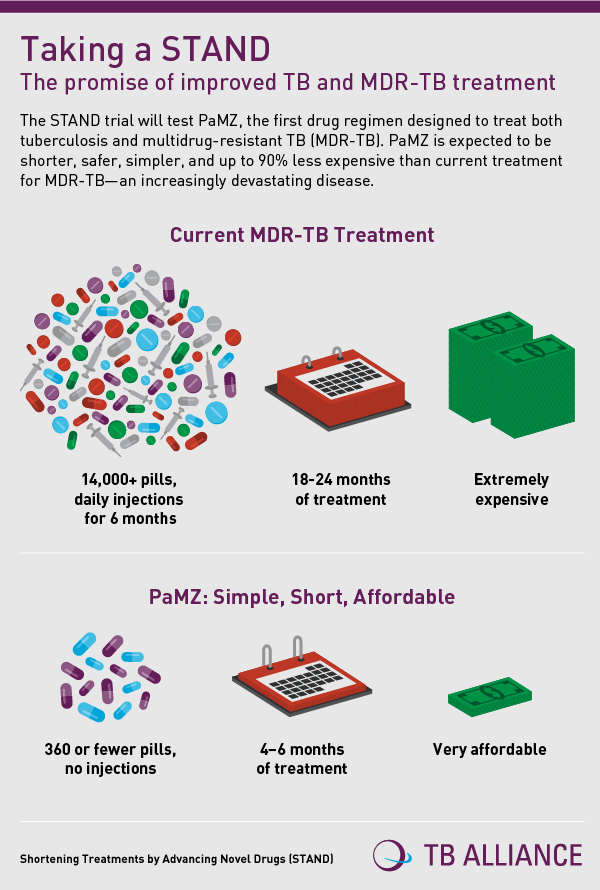
TB kills an estimated 1.5 million people annually, and is one of the world’s most deadly diseases. Currently, people with MDR-TB require 18 to 24 months of treatment, with thousands of pills and daily injections for at least six months.
Read: New drug available for TB patients
According to the World Health Organisation, of the 480,000 patients with MDR-TB in 2013, only 20 percent were treated. Of those treated, less than half (48 percent) of MDR-TB patients were cured.
Even those with drug-sensitive TB require a minimum of six months of therapy, a significant burden for people with TB and the programmes that care for them.
“Today’s TB treatment options are inadequate compared to the scale of the problem, and the inadequacies can overwhelm patients, families, and health systems,” said Mel Spigelman, MD, President and CEO of TB Alliance, the trial’s sponsor.
First regimen designed to treat drug-sensitive and MDR-TB
“PaMZ is the first regimen designed to treat drug-sensitive and MDR-TB. As such, it could help ensure TB patients and healthcare providers have a more effective treatment option to help in scaling up the response to combatting the disease.”
The STAND trial will test PaMZ, a three-drug regimen comprised of two candidate drugs that are not yet licensed for use against TB – pretomanid (previously known as PA-824) and moxifloxacin – and one approved TB antibiotic, pyrazinamide.
The new therapy is promising for those patients with active TB and whose organisms are sensitive to the three drugs. This includes one-third to one-half of all MDR-TB cases as well as those classically defined as having drug-sensitive TB. Additionally, PaMZ shows promise to improve treatment for those patients co-infected with HIV.
Read: Drug-resistant TB remains a crisis
“The results of this trial show the potential for the PaMZ regimen to improve treatment for tuberculosis,” says Rod Dawson, MD, head of the Centre for TB Research Innovation at the University of Cape Town, South Africa, and lead author of the paper detailing the results of the PaMZ regimen published in The Lancet.
"In our initial study we noted that the new combination is up to two times more effective than the current regimes based on spit testing at eight weeks," Dawson told Health24 resident doctor, Dr Owen Wiese.
“Especially important is that PaMZ may have a unique application as a potentially shorter, injection-free regimen for a select sub-group of patients with MDR-TB.”
When asked about the specific combination of drugs, Dawson said that Pretomanid is a highly active new synthetic drug found to have very limited interaction with other medications. "This is important where a patient is already taking other medication like antiretroviral treatments." Dawson explains that Moxifloxacin is a well known antibiotic, long used for treating chest infections but was not previously licensed for use in the treatment of drug sensitive TB. Pyrozinamide, Dawson says, is an existing component of current TB treatment regimes and is essential in preventing relapse after the treatment is complete.
1,500 patients in 15 countries across Africa
STAND researchers expect to enroll 1,500 patients in 15 countries across Africa (Kenya, South Africa, Tanzania, Uganda, Zambia), Asia (China, Malaysia, Philippines, Thailand), Caribbean (Haiti), Eastern Europe (Georgia, Russia, Ukraine), and Latin America (Brazil, Peru) in this study.
PaMZ will be tested in STAND as a 4- and 6-month treatment for drug-sensitive TB and a 6-month treatment for drug-resistant TB, and also enroll those co-infected with HIV.
"We expect to start seeing results at 2 months after initiation of the treatment," Dawson said to Dr Wiese.
Each patient will be followed for two years starting from the beginning of treatment. The STAND trial partners with many of the communities in which the study is conducted through its robust community engagement programme.
"It is very important to find new drugs and treating regimes with a shorter treatment duration to tackle the TB problem," Dawson said to Wiese. "We have been using the same drugs for TB treatment since the 1970's. We need new combinations."
Read: Is TB spinning out of control in South Africa?
If successful in this Phase 3 trial, the PaMZ regimen would eliminate the need for injectable drugs and reduce the cost of MDR-TB therapy by more than 90 percent in those patients whose TB organisms are sensitive to the three drugs.
It also promises to be compatible with commonly used HIV drugs, helping the millions of people co-infected with TB/HIV.
Read More:
Resources out of rural patients’ reach
SA in dark on drug resistant TB
Image from Shutterstock




 Publications
Publications
 Partners
Partners