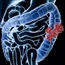
Three of every 20 flexible endoscopes used to examine patients' gastrointestinal tracts and colons were improperly cleaned, a new study finds.
Those 15% of endoscopes had unacceptable levels of "bio dirt" - cells and matter from a patient's body that could pose a potential infection risk to other patients, according to the researchers.
They examined 275 flexible duodenoscopes, gastroscopes, and colonoscopes used at five US hospitals and found that 30%, 24% and 3%, respectively, did not pass a cleanliness rating.
The study findings were to be presented last weekend at the annual meeting of the Association for Professionals in Infection Control and Epidemiology (APIC).
"Three out of 20 is an unexpectedly high number of endoscopes failing a cleanliness criterion," lead investigator Marco Bommarito, lead research specialist at 3M Infection Prevention Division, said in an APIC news release. "Clearly, we'd like no endoscopes to fail a cleanliness rating."
Patients having to check for HIV
In recent years, improperly cleaned endoscopes at medical facilities in the United States have resulted in thousands of patients having to be checked for HIV and hepatitis B and C, according to the news release. More health-care-associated outbreaks have been linked to contaminated endoscopes than to any other medical device, the US Centers for Disease Control and Prevention has reported.
Each year in the United States, between 15 million and 20 million endoscopy procedures are conducted with reusable endoscope devices to screen various parts of patients' gastrointestinal tracts and look for problems such as cancer.
Duodenoscopes examine the duodenum (the first section of the small intestine), while gastroscopes examine the stomach and colonoscopes examine the colon.
"The cleaning protocols for flexible endoscopes need improvement, such as guidelines tailored to the type of scope or identifying if there is a critical step missing in the manual cleaning process, and documented quality-control measures," Bommarito said. "These types of improvements could have a positive impact on patient safety."
Because this study was presented at a medical meeting, the data and conclusions should be viewed as preliminary until published in a peer-reviewed journal.
More information
The American Cancer Society has more about endoscopy.




 Publications
Publications
 Partners
Partners