This article has not necessarily been edited by Health24.
WEDNESDAY, Sept. 15 (HealthDay News) -- Krystexxa (pegloticase) has been approved by the U.S. Food and Drug Administration for adults with gout who do not respond to, or who cannot tolerate, standard treatments.
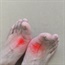
Gout results when the body produces too much uric acid, which crystallizes in the joints or soft tissue. This leads to symptoms including pain, stiffness, swelling and redness.
The condition has been strongly associated with other health issues including obesity, high blood pressure, high cholesterol and diabetes, the FDA said in a news release.
Krystexxa, given by injection every two weeks, metabolizes uric acid into a harmless byproduct that is excreted in the user's urine, the agency said. The drug's safety and effectiveness were established in a pair of six-month clinical trials involving a total of 212 people.
The FDA warned that since severe allergic reactions were a common side effect of Krystexxa, a corticosteroid and antihistamine should be given beforehand to minimize this risk. Other common adverse reactions have included gout flares, nausea, bruising at the injection site, nasal irritation, constipation, chest pain and vomiting.
The agency said Krystexxa hadn't been evaluated in people with congestive heart failure, and should be dispensed to such users with caution.
The drug is produced by Savient Pharmaceuticals, based in East Brunswick, N.J.
More information
To learn more about gout, visit U.S. National Library of Medicine.
Read more:
Causes of Gout
Treatment of Gout
(Copyright © 2010 HealthDay. All rights reserved.)




 Publications
Publications
 Partners
Partners